Ligand Interactions
Presentations | English
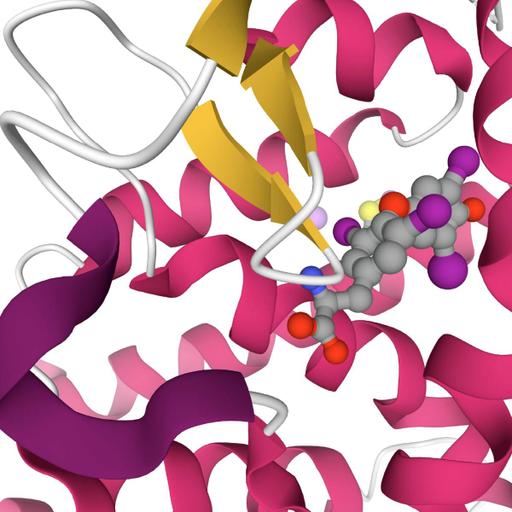
An ion or molecule can be called as a ligand. Its capable of donating electrons to a central atom and forming a coordination complex. Their classifications are based on number of binding sites with the central metal atom, their size and charges. The bonding between a ligand and metal ion is ionic or covalent. The binding site and ligand interaction depends on binding affinity. A protein ligand combination is a non-covalent bond which is reversible reaction. This is very important for all processes in living organism. Types include reversible binding, allosteric protein, cooperative binding and complementary binding. Allosteric is the process of ensuring oxygen binding to protein. Cooperative reaction can be explained as oxygen binding to heamoglobin. Two or more ligands binding with the same protein cause mutual interaction. This reaction changes different functions of proteins. More details can be obtained from the presentation.
Free
PPTX (17 Slides)
Ligand Interactions
Presentations | English
