Hydrogen Bonds in Water and Ice
Presentations | English
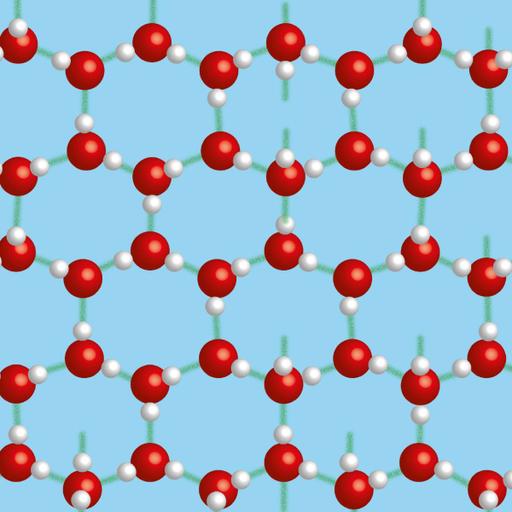
A hydrogen bond is a type of attractive intermolecular force that exists between two partial electric charges of opposite polarity. It is stronger than other intermolecular forces, but weaker than ionic and covalent bond. The hydrogen must be attached to a strongly electronegative heteroatom such as oxygen, nitrogen or fluorine which is called the hydrogen-bond donor in a hydrogen bond. The most ubiquitous examples of a hydrogen bond are found between water and ice molecules. In ice, each water molecule is hydrogen bonded to 4 other neighboring water molecules to form a crystal. This arrangement is necessary to ensure the strongest degree of hydrogen bonding in a uniform crystal lattice resulting in ice structure. In liquid water, each molecule is hydrogen bonded to approximately 3.4 other water molecules. These hydrogen bonds are constantly being formed and breaking up because of rapid thermal motions of molecules, but in ice hydrogen bonds is stable.
Free
PPTX (40 Slides)
Hydrogen Bonds in Water and Ice
Presentations | English
