Free Radicals
Presentations | English
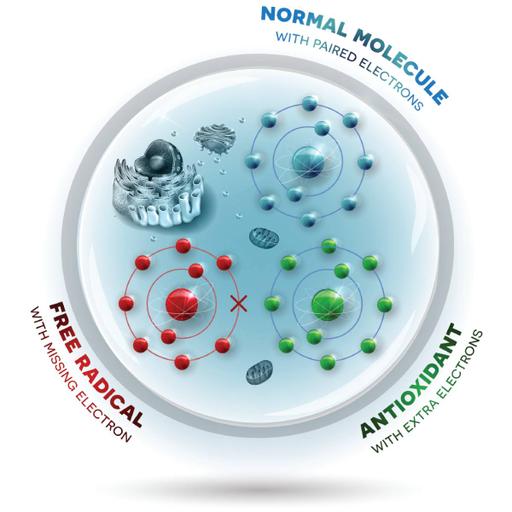
A molecule with at least one unpaired electron is referred to as a radical or free radical in chemistry. Even quantities of electrons are found in most compounds. Molecules that include oxygen and have an unbalanced number of electrons are known as free radicals. They can easily interact with other molecules because of their unequal quantity. These atoms interact with other molecules so readily that they might trigger lengthy chemical chains in your body. These processes are referred to as oxidations. The electrons that surround atoms orbit them in layers known as shells. There must be a specific number of electrons in each shell. As oxygen molecules fragment into single unpaired electron atoms, the resulting free radicals are unstable and seek out other atoms or molecules to form bonds with. For more information browse this PPT.
25.75
Lumens
PPTX (103 Slides)
Free Radicals
Presentations | English
