First Law of Chemical Thermodynamics
Presentations | English
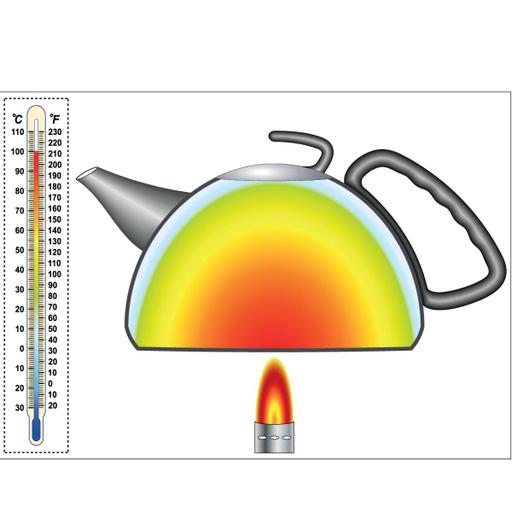
Is it true that The law of conservation of energy and the First law of thermodynamics are the same? Yes, the First Law of Thermodynamics, is also termed as the law of conservation of energy, states that energy can be neither created nor destroyed—it can only be transformed from one form to another (the total energy remains constant). The first law of thermodynamics, arguably the most important, is an expression of the principle of conservation of energy. The limitation of the first law of thermodynamics is that it does not say anything about the direction of the flow of heat. It does not say anything whether the process is spontaneous or not. The reverse process is not possible. In actual practice, the heat doesn't convert completely into work. The most common practical application of the First Law is the heat engine. Heat engines convert thermal energy into mechanical energy and vice versa. The water is converted to steam, and the pressure is then used to drive a piston that converts heat energy to mechanical energy.
5.50
Lumens
PPTX (22 Slides)
First Law of Chemical Thermodynamics
Presentations | English
