Atomic Structure
Presentations | English
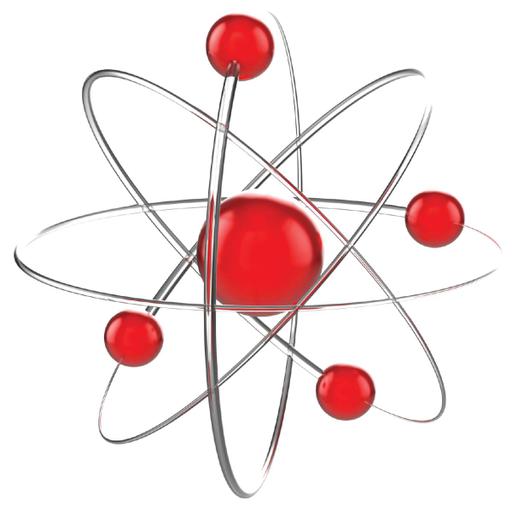
The history of atomic structure and quantum mechanics dates back to the times of Democritus, the man who first proposed that matter is composed of atoms. Atomic structure refers to the structure of an atom comprising a nucleus in which the protons and neutrons are present. The negatively charged particles called electrons revolve around the centre of the nucleus. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus. Neutral atoms have equal numbers of protons and electrons. Nucleons are the components of the nucleus of an atom. A nucleon can either be a proton or a neutron. Each element has a unique number of protons in it, which is described by its unique atomic number. However, several atomic structures of an element can exist, which differ in the total number of nucleons.
8.00
Lumens
PPTX (32 Slides)
Atomic Structure
Presentations | English
